The solubility of hydrogen sulfide hydrate in water
Recently, Dr. Chuanjun Wu and Dr. Jiangzhi Chen from the Department of Experimental Study under Deep-sea Extreme Conditions published a paper entitled with "Experimental Determination and Thermodynamic Modeling of the Hydrogen Sulfide Hydrate Solubility in Water". This paper was supported by the National Natural Science Foundation of China (grant numbers: 41873068, 41804085, and 41973055), and the professor Jiang Lei is the corresponding author. The link to this paper is: https://doi.org/10.1016/j.ces.2023.119474.
The solubility of gas hydrates, referring to the concentration of dissolved gas in water in equilibrium with hydrate, is necessary to maintain hydrate stability. Hydrogen sulfide (H2S) is an important acid gas that occurs naturally, such as H2S-bearing gas reservoirs in southwest China, Wyoming state in the United States and Alberta in Canada. H2S is prone to hydrate formation at low temperatures and high pressures, resulting in pipeline plugging and subsequent pressure drop during natural gas transportation and operation. Therefore, it is critical to obtain the stability conditions of H2S hydrate in water. Also, it requires an evaluation of the dissolved H2S content in water while using hydrate method for separating H2S from natural gas. To address this issue, a high-pressure optical cell (HPOC) coupled with a Raman spectrometer was used to determine the solubility of H2S hydrate at a temperature of 273.15–301.15 K and pressures ranging from the hydrate–liquid–vapor (H–Lw–V) three-phase equilibrium pressure for each temperature to 100 MPa based on the principle that the content of dissolved H2S in aqueous solution is proportional to the Raman peak area under the same temperature and pressure conditions.
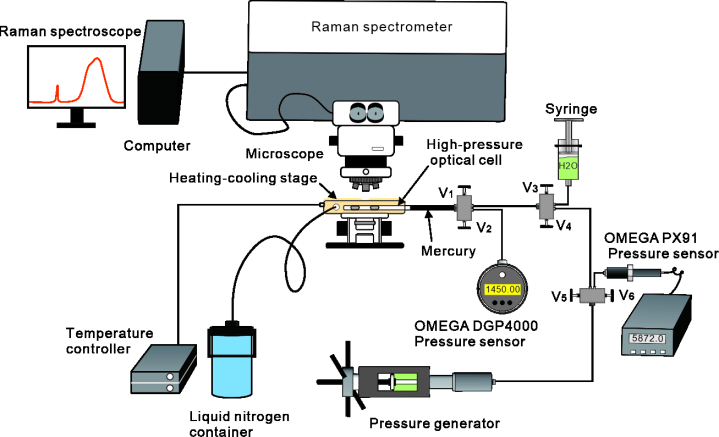
Schematic of the experimental system showing the collection of Raman spectra for dissolved H2S and water
The results showed that the solubility of H2S hydrate in water increases with temperature under H–Lw–V three-phase equilibrium conditions. Meanwhile, under hydrate–liquid two-phase equilibrium conditions, the solubility increases significantly with temperature at constant pressure but decreases slightly with pressure at constant temperature. The three-phase solubility can be expressed as mH2S(mol·kg-1)=exp(-6176.6286/T+20.8318)(R2=0.9975), where the temperature is in K, and the two-phase solubility also depends on the pressure in MPa: mH2S(mol·kg-1)=exp[-21.1-0.0182P-(6281.47-4.23P)/T](R2=0.9976).
In order to expand the application scope of the results, a thermodynamic model was developed based on the van der Waals-Plateeuw theory (in short vdW-P model). For calculation of the chemical potential of water in hydrate, the Kihara potential energy function was used to calculate the Langmuir constant that quantifies the tendency of hydrate cavity to adsorb H2S molecules, and the model Duan (2007) was employed to calculate the fugacity coefficient of H2S. Additionally, the Holder model (1980) was used to calculate the chemical potential of water in the liquid phase, and the solubility of H2S in water was adopted by the model proposed by Jiang (2020). The results showed that the general deviation between the model and the experimental data is within 10%, indicating that the model has a relatively good predictive ability.
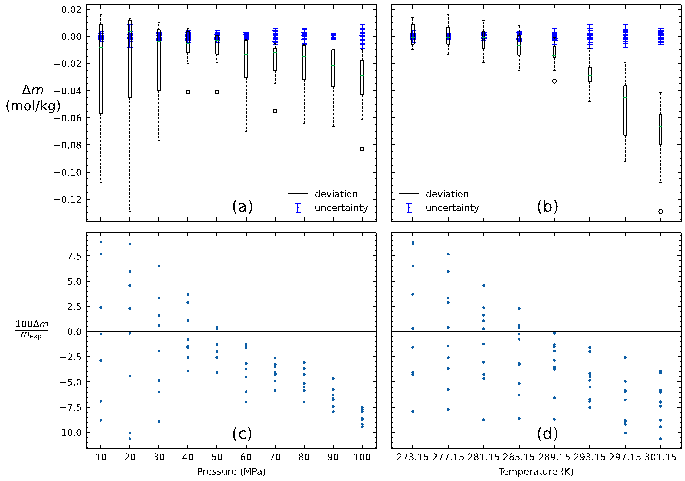
Measurement uncertainties of the simulations by pressure (a and c) and temperature (b and d), excluding the H–Lw–V measurements